
Sorry, Out of Stock
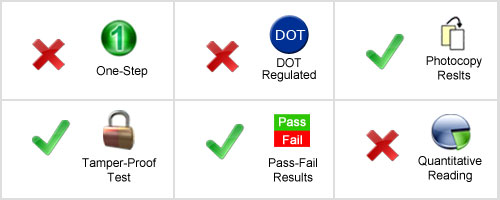
Be sure to be legal: Positive instant employee alcohol tests should only be used to attain preliminary results that allow for quick screening of positives from negatives. Note that instant employee Alcohol testing kits cannot be sent in for Confirmation or Confirmation with Medical Review Officer Services (MRO). To stand up in a court of law we recommend a new test be performed by a certified third party onsite collection service or by utilizing a certified offsite employee drug testing service at one of our 12,000+ locations.
How To Use: User needs to blow into the drug testing device for 12 seconds. Within 2 minutes after exposure to breath the drug test device provides a pass/fail reading. A tube contains crystals that change color in only 2 minutes upon exposure to breath, indicating alcohol presence – with color ranges from yellow to blue indicating over the legal DUI limit for different states and countries. This employee drug test is available in 0.02%, 0.05% and 0.08%.
Manufacturer: Breath Scan Alcohol tests are manufactured by Akers Biosciences, Inc. ABI is headquartered in North America in Thorofare, New Jersey USA, with a European office located in London, England. Since 1989, ABI's team of Research and Development specialists has taken an aggressive approach to advancing the science of diagnostics while responding to major shifts in health care.
Sorry there is no information here for this product.
Please try another Tab.
Product Information
FDA 510k Clearance Documents
If you are in need of the 510k Certification for this product, please contact us and will forward the correct certificates to you.
Institutions Homepages
Glossary Information